Benezyme
$ 30.00
Size: 60 Vegetarian Capsules
Premier Enzyme Blend for Better Digestion and Relief from Gas and Bloating*
Please select all options.
Even with a healthy diet, many factors in our modern lives such as stress, dysbiosis, eating on the go, and even aging can interfere with optimal digestion, depleting our nutrient levels.*
The plant-based enzymes in Benezyme can assist in the breakdown and absorption of a broad spectrum of nutrients, allowing us to get the most benefit out of our food.*
Product Highlights:
- Aids in the healthy digestion of carbohydrates, proteins, and fats*
- Helps break down polysaccharides in legumes that can be associated with gas and bloating*
- Supports the digestion of fibers from fruits and vegetables to help reduce GI discomfort*
- Improves nutrient metabolism by helping break down phytates, compounds found in grains and seeds that interfere with mineral absorption*
- Supports lactose intolerance by helping to break down lactose, the difficult-to-digest sugar found naturally in dairy products*
- Inhibits the growth of Candida and intestinal parasites*
Unlike porcine-based enzymes, Benezyme is active across a wide pH range, which means it can maintain potency through the strong acidity of the stomach. It’s easy to see why Benezyme is our patients’ favorite digestive aid!
*These statements have not been evaluated by the FDA and are not intended to treat or cure any disease.
We love this enzyme blend because it covers all the important functions our patients need to optimize their digestion.
Benezyme has a strong emphasis on protein-digesting enzymes like proteases, peptidase, papain, and bromelain because optimal protein digestion is necessary for muscle growth, hormone function, and skin health, while our ability to break down protein naturally declines with age.1 We find a lot of our patients can use a little support with digesting their protein!
But this supplement also has a comprehensive array of enzymes for breaking down fiber and resistant polysaccharides from plant foods: amyloglucosidase, cellulase, hemicellulase, alpha-galactosidase, pectinase, xylanase, beta-glucanase, and phytase. This broad-spectrum blend helps support maximum nutrient absorption and prevent overfeeding gas-producing bacteria in the gut. In our experience, this can be an important first step towards healing the gut.
Several of these enzymes even support a healthy balance of gut microflora. Papain from papaya and bromelain from pineapple can inhibit the growth of intestinal parasites,2 and beta-glucanase can reduce resistant biofilms of the fungus Candida in the gut.*[3]
*These statements have not been evaluated by the FDA and are not intended to treat or cure any disease.
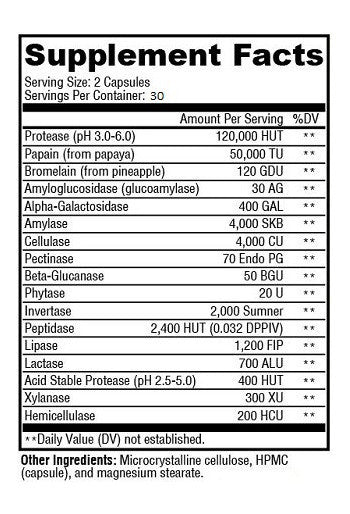
2 CAPSULES PER SERVING
Protease (pH 3.0-9.0) 120,000 HUT
Papain (from papaya) 50,000 TU
Bromelain (from pineapple) 120 GDU
Amylase 4,000 SKB
Amyloglucosidase (glucoamylase) 30 AG
Cellulase 4,000 CU
Beta-Glucanase 50 BGU
Alpha-Galactosidase 400 GAL
Invertase 2,000 Sumner
Peptidase (29 DPPIV) 2,400 HUT
Pectinase 70 Endo PG
Lactase 700 ALU
Phytase 20 U
Acid Stable Protease (pH 2.0-3.5) 400 HUT
Lipase 1,200 FIP
Xylanase 300 XU
Hemicellulase 200 HCU
Other Ingredients: HPMC (capsule), microcrystalline cellulose, stearic acid, magnesium stearate, and silica.
FORMULATED TO EXCLUDE: Wheat, gluten, yeast, soy, animal or dairy products, fish, shellfish, peanuts, tree nuts, egg, GMOs, artificial colors, or artificial sweeteners.
Take 1-2 capsules daily, or use as directed by your healthcare practitioner. If necessary, capsules may be opened and contents sprinkled over food.
Background: Dietary protein ingestion stimulates muscle protein synthesis by providing amino acids to the muscle. The magnitude and duration of the postprandial increase in muscle protein synthesis rates are largely determined by dietary protein digestion and amino acid absorption kinetics.
Objective: We assessed the impact of protein type, protein dose, and age on dietary protein digestion and amino acid absorption kinetics in vivo in humans.
Methods: We included data from 18 randomized controlled trials with a total of 602 participants [age: 53 ± 23 y; BMI (kg/m2): 24.8 ± 3.3] who consumed various quantities of intrinsically l-[1-13C]-phenylalanine-labeled whey (n = 137), casein (n = 393), or milk (n = 72) protein and received intravenous infusions of l-[ring-2H5]-phenylalanine, which allowed us to assess protein digestion and phenylalanine absorption kinetics and the postprandial release of dietary protein-derived phenylalanine into the circulation. The effect of aging on these processes was assessed in a subset of 82 young (aged 22 ± 3 y) and 83 older (aged 71 ± 5 y) individuals.
Results: A total of 50% ± 14% of dietary protein-derived phenylalanine appeared in the circulation over a 5-h postprandial period. Casein ingestion resulted in a smaller (45% ± 11%), whey protein ingestion in an intermediate (57% ± 10%), and milk protein ingestion in a greater (65% ± 13%) fraction of dietary protein-derived phenylalanine appearing in the circulation (P < 0.001). The postprandial availability of dietary protein-derived phenylalanine in the circulation increased with the ingestion of greater protein doses (P < 0.05). Protein digestion and phenylalanine absorption kinetics were attenuated in older when compared with young individuals, with 45% ± 10% vs. 51% ± 14% of dietary protein-derived phenylalanine appearing in the circulation, respectively (P = 0.001).
Conclusions: Protein type, protein dose, and age modulate dietary protein digestion and amino acid absorption kinetics and subsequent postprandial plasma amino acid availability in vivo in humans. These trials were registered at clinicaltrials.gov as NCT00557388, NCT00936039, NCT00991523, NCT01317511, NCT01473576, NCT01576848, NCT01578590, NCT01615276, NCT01680146, NCT01820975, NCT01986842, and NCT02596542, and at http://www.trialregister.nl as NTR3638, NTR3885, NTR4060, NTR4429, and NTR4492.
The relative anthelmintic efficacy of plant-derived cysteine proteinases on intestinal nematodes
We examined the in vitro and in vivo efficacy of plant cysteine proteinases (CPs) derived from pineapple (Ananas comosus) and kiwi fruit (Actinidia deliciosa), and compared their efficacy as anthelmintics to the known effects of CPs from the latex of papaya (Carica papaya) against the rodent intestinal nematode, Heligmosomoides bakeri. Both fruit bromelain and stem bromelain had significant in vitro detrimental effects on H. bakeri but in comparison, actinidain from kiwi fruit had very little effect. However, in vivo trials indicated far less efficacy of stem bromelain and fruit bromelain than that expected from the in vitro experiments (24.5% and 22.4% reduction in worm burdens, respectively) against H. bakeri. Scanning electron microscopy revealed signs of cuticular damage on worms incubated in fruit bromelain, stem bromelain and actinidain, but this was far less extensive than on those incubated in papaya latex supernatant. We conclude that, on the basis of presently available data, CPs derived from pineapples and kiwi fruits are not suitable for development as novel anthelmintics for intestinal nematode infections.