NAC
$ 34.00
Size: 60 Vegetarian Capsules
Specialized Amino Acid for Immunity and Antioxidant Support
Please select all options.
N-Acetyl Cysteine (NAC) supplies the conditionally essential amino acid L-cysteine, a precursor to the super antioxidant glutathione. Together, NAC and glutathione support the body’s antioxidant and detoxification activity, providing powerful benefits for a wide range of conditions.* NAC also helps to break down excess mucus and act as a protective shield during cold and flu season.*[1,2]
Highlights:
- Replenishes Glutathione Stores*
- Protects Tissues from Oxidative Stress*
- Supports Detoxification of Environmental Toxins and Pollutants*
- Supports Antioxidant Activity in all Body Cells*
- Protects Against Liver Damage*
- May Help Reduce Inflammation*
- Supports Mitochondrial Function*
- Supports Healthy Metabolism and Insulin Sensitivity*
- Promotes Healthy Respiratory Function*
Hundreds of clinical trials have been performed with NAC, demonstrating countless benefits throughout numerous body systems. NAC has been shown to reduce symptoms of depression,3 as well as support numerous psychiatric and neurological disorders including brain healing after concussion.*[4,5] NAC is a potent anti-inflammatory, and has been found to improve joint pain in rheumatoid arthritis.*[6] NAC protects the liver and mitochondria from damage by certain drugs and antibiotics.*[7-9] NAC is also a potent biofilm buster, making it a powerful tool against chronic infections.*[10]
*These statements have not been evaluated by the FDA and are not intended to treat or cure any disease.
NAC is a Morrison Center favorite for helping to get your health back on track.* With benefits for promoting detoxification, protecting against oxidative stress, reducing inflammation, supporting the mitochondria, strengthening immunity, and boosting metabolism, NAC is a powerful component of many effective protocols for increasing the body’s natural ability to heal.
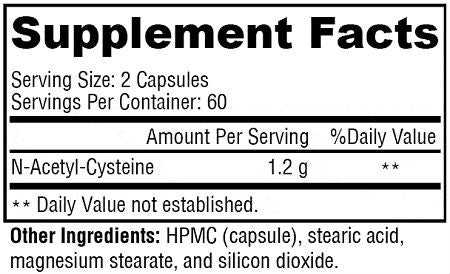
2 Capsules per serving
N-Acetyl-L-Cysteine 1.2 g
Other Ingredients: HPMC (capsule), stearic acid, magnesium stearate, and silica
FORMULATED TO EXCLUDE: Wheat, gluten, corn, yeast, soy, animal and dairy products, fish, shellfish, peanuts, tree nuts, egg, GMOs, artificial colors, artificial sweeteners, and artificial preservatives.
Take one to two capsules twice daily between meals, or as directed by your healthcare practitioner.
Caution: Individuals taking medication should discuss potential interactions with their healthcare practitioner
Objective: This meta-analysis was performed to assess the possible prophylactic benefit of prolonged treatment with oral N-acetylcysteine (NAC) in chronic bronchitis (CB) based on qualifying clinical trials. Treatment of acute exacerbations with NAC was not investigated.
Background: Prolonged treatment with oral NAC has been investigated in a number of studies of patients with CB. NAC prevented acute exacerbations and symptoms of CB in some but not all trials.
Methods: The trials included in this analysis were selected from a MEDLINE search of the period from January 1, 1980, through June 30, 1995; references in the articles retrieved in the initial search; and consultation with 2 experts. Selection was based on the following criteria: published, double-blind, placebo-controlled, chronic bronchopulmonary disease, duration of therapy > or =2 months, and data sufficient to calculate an outcome variable permitting direct comparison of studies (effect size) for both NAC and placebo groups. The primary end point was the incidence of acute exacerbations in 7 of 8 trials and clinical assessment in the other. In 7 studies, inclusion criteria were based on Medical Research Council criteria for CB, with an additional criterion in some trials. For the meta-analysis, the end points of individual trials were transformed into an effect size as a common outcome.
Results: Of 21 trials initially identified, 8 qualified for inclusion. References from the 8 papers and consultation with the experts produced 8 additional publications, 1 of which qualified for inclusion. NAC was administered orally at a daily dose of 400 mg (1 study), 600 mg (5 studies), or 1200 mg (1 study). One other trial used a dose of 600 mg 3 times per week. The duration of treatment was 3 months (1 study), > or =5 months (2 studies), or 6 months (7 studies). The results of this meta-analysis showed a statistically significant effect size for NAC compared with placebo. The overall value of effect size was -1.37 (95% CI, -1.5 to -1.25). Sensitivity analyses did not significantly alter these results. In a subset analysis of trials with the number of acute exacerbations as a clinical end point, a mean difference of -0.32 clinical event (95% CI, -0.50 to -0.18) was found (ie, a 23% decrease in the number of acute exacerbations compared with placebo).
Conclusion: These findings suggest that a prolonged course of oral NAC prevents acute exacerbations of CB, thus possibly decreasing morbidity and health care costs.
N-acetylcysteine (NAC), an analogue and precursor of reduced glutathione, has been in clinical use for more than 30 yrs as a mucolytic drug. It has also been proposed for and/or used in the therapy and/or prevention of several respiratory diseases and of diseases involving an oxidative stress, in general. The objective of the present study was to evaluate the effect of long-term treatment with NAC on influenza and influenza-like episodes. A total of 262 subjects of both sexes (78% > or = 65 yrs, and 62% suffering from nonrespiratory chronic degenerative diseases) were enrolled in a randomized, double-blind trial involving 20 Italian Centres. They were randomized to receive either placebo or NAC tablets (600 mg) twice daily for 6 months. Patients suffering from chronic respiratory diseases were not eligible, to avoid possible confounding by an effect of NAC on respiratory symptoms. NAC treatment was well tolerated and resulted in a significant decrease in the frequency of influenza-like episodes, severity, and length of time confined to bed. Both local and systemic symptoms were sharply and significantly reduced in the NAC group. Frequency of seroconversion towards A/H1N1 Singapore 6/86 influenza virus was similar in the two groups, but only 25% of virus-infected subjects under NAC treatment developed a symptomatic form, versus 79% in the placebo group. Evaluation of cell-mediated immunity showed a progressive, significant shift from anergy to normoergy following NAC treatment. Administration of N-acetylcysteine during the winter, thus, appears to provide a significant attenuation of influenza and influenza-like episodes, especially in elderly high-risk individuals. N-acetylcysteine did not prevent A/H1N1 virus influenza infection but significantly reduced the incidence of clinically apparent disease.